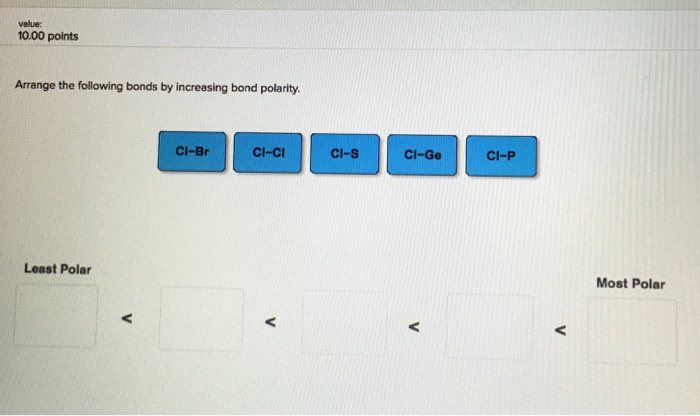
Arrange the Following Bonds by Increasing Bond Polarity.
Email me if my answer is selected or commented on. The enthalpy of a reaction ΔHrxn is the overall heat change resulting from ______ energies when reactant bonds break and ______ energies when product bonds form.

Solved Question 13 Arrange The Following Bonds In Order Of Chegg Com
H-S H-P.
. Arrange the following bonds in order of increasing polarity. Find the difference in electronegativity for each atom in the bond. Email me at this address if my answer is selected or commented on.
Arrange the following bonds from least polar to most polar. Cs to F Cl to Cl Br to Cl Si to C. Video Player is loading.
C-H N-H O-H F-H. Ill give you the general steps and outline how to do it but this is something you should do yourself so that you get how to do it. Larger difference more polar thus the more electronegative atom hogs the electron density more and vice.
And they can bond with hydro filic substances for example water here. First calculate the electronegativity difference E between atoms in each bond. The sum of the bonds formed exothermic.
A H- Cl bond E 21 30 09. Read the notes below. Arrange the given bonds in increasing order of polarity.
H has a low electronegativity then it comes C O and F so the order of increasing of polarity is. Arrange the following bonds in order of increasing bond polarity. Arrange the following bonds in order of increasing polarity.
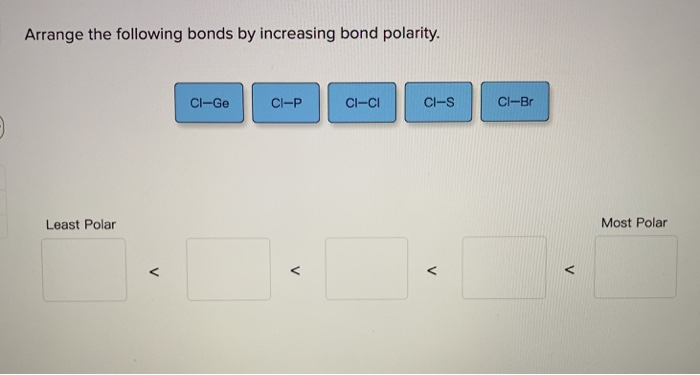
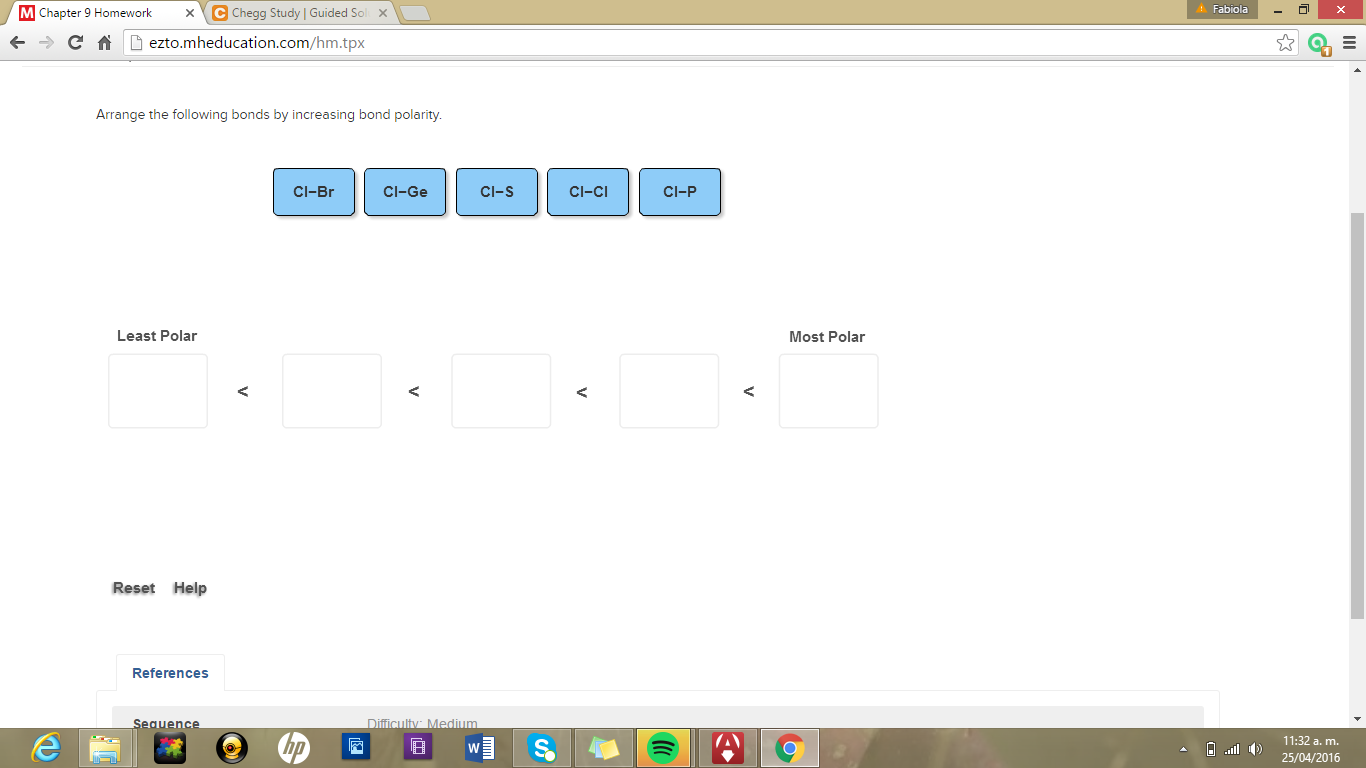
Smartbook Chapter 9 Part 2. The KI bond in KI Q11. Cl-S Cl-P Cl-Si Cl-Cl.
The CC bond in H 3 CCH 3 Q10. H-F H-N H-O H-Cl arrow_forward Rank these bonds in order of increasing polarity. See the answer See the answer done loading.
This problem has been solved. Then arrange the bonds from smallest E value to greatest _E value. A mathrm HCl b mathrm PH_ 3 c mathrm H_ 2 mathrm O d mathrm CF_ 4 Problem.
A OH CH HH FH. The polarity in the molecules is due to the difference in the electronegativities constituting atoms and symmetry of the molecule there is no electronegative difference. H-P.
So essentially the easiest way to determine whether or not chemical bonds or capable of polarity is if they are highly electro negative. Arrange the substances with polar covalent bonds in order of increasing bond polarity. H-Cl H-I H-Br H-F.
Arrange the flowing bonds according to increasing bond polarity. Arrange the following bonds in order of increasing polarity. A Cl-S.
A F-H b S-H c P-H. Arrange the bonds in each of the following sets in order. So lets go through the options.
So when the bond is between the same element the polarity is 0 such as H-H. A KCl b P 4 c B F 3 d S O 2 e B r 2 f N O 2. The bigger the difference between oxygen and the other atoms electronegativity values the more polar the bond will be.
P - H H - O N - H H - F. SOLVEDArrange the following molecules in order of the increasing polarity of their bonds. Which of the following would be a polar molecule.
A CF OF BeF. Arrange the following molecules by increasing bond polarity. Look up the electronegativities of each atom in your textbook.
Use electronegativity difference to arrange the following bonds in order of increasing polarity. Classify the following bonds as ionic polar covalent or covalent. H-N H-O H-P H-S D.
Arrange the following bonds in order of increasing polarity. The anion in an ionic compound whose name ends in ide is. H-P H-S.
Ammonia N H 3 and phosphorus trihyd. B OCl SBr CP. This means that in order of increasing polarity you can arrange these bonds like this colorgreenCl-O C-O P-O Alternatively you can consult the actual electronegativity values and confirm that weve got the order right.
The arrangement of by increasing bond polarity. The shape of a BF3 molecule is. Select the statement that correctly summarizes the steps to calculate ΔHo of a reaction using bond enthalpies.
Arrange the following bonds in order of increasing polarity. Both polar bonds and an unsymmetrical arrangement. The Octet Rule Noble gases like He Ne Ar Kr etc are.
Hence it is nonpolarthere is electronegative difference between C-Cl but it is a symmetrical molecule. A H Cl b C-O c N-F d Si- I and e O O. C CS BF NO.
H-O. Cl2 CaCl2 CsCl PCl3 CCl4. Developing mastery Lets Study This.
So the electronegativity increases from the bottom to top and from the left to right in the periodic table. So the question here wants us to rank the following chemical bonds in increasing order of polarity here.
Solved Arrange The Following Bonds By Increasing Bond Chegg Com
Solved Arrange The Following Bonds By Increasing Bond Chegg Com

Solved Value 10 00 Points Arrange The Following Bonds By Chegg Com
Comments
Post a Comment